Grade 12 Standard electrode potentials in PowerPoint
R15.18
Use, by you or one client, in a single end product which end users are not charged for. The total price includes the item price and a buyer fee.
Resource Description
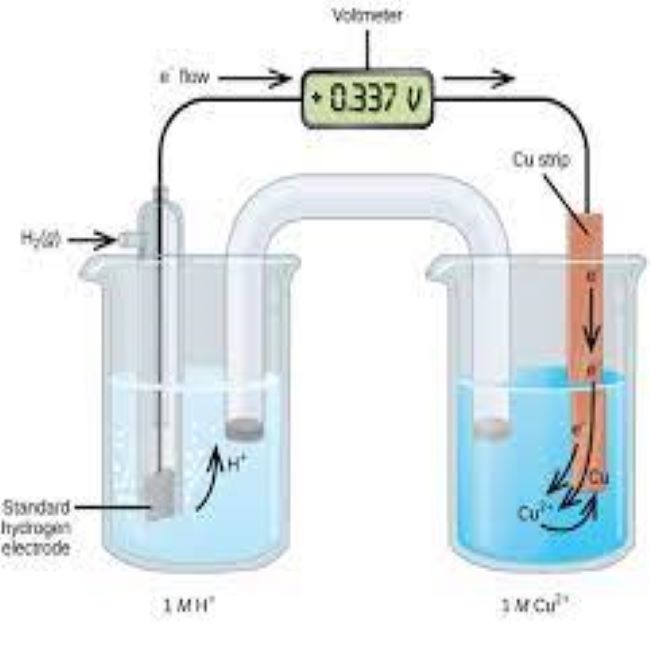
- Components of the standard half cell
- Value of standard hydrogen half cell
- Operating conditions for the standard half cell
- Measuring standard half cell potentials for other half cells
- Hydrogen-Zinc electrochemical cell to establish electrode potential value for the Zn half cell
- Redox potential table & order in table
- Using the redox potential table
- Determining the emf of a cell
- Symbolic representation of a cell
- Predicting whether redox reactions are spontaneous
- Exercises
- Balancing equations using the half reaction method



 KES(KSh)
KES(KSh) USD($)
USD($) GBP(£)
GBP(£) GHS(₵)
GHS(₵) NGN(₦)
NGN(₦) MUR(₨)
MUR(₨) BWP(P)
BWP(P) AUD($)
AUD($) TZS(Sh)
TZS(Sh) INR(₹)
INR(₹) PHP(₱)
PHP(₱) AED(د.إ)
AED(د.إ)